Make Plans Now to Attend the 2018 Annual Canadian Educational Meeting!
 The Quinism Foundation will be hosting its first-ever annual Canadian educational meeting a little over a month from now, in beautiful Kanata, Ontario, just outside the nation’s capital. Make plans now to join us at the Comfort Inn Ottawa West the afternoon of Tuesday, September 18, 2018 for a series of talks focused on the concerns of Canadian mefloquine veterans and Canadian healthcare providers caring for these veterans. Topics will include an update on the status of current VAC claims related to mefloquine poisoning and factors to consider when filing a VAC claim, including those conditions that can be linked to mefloquine exposure. The meeting will also discuss use of the foundation’s WRMI-2 screening instrument for symptomatic mefloquine exposure, and how clinicians and veterans can improve documentation supporting their claim.
The Quinism Foundation will be hosting its first-ever annual Canadian educational meeting a little over a month from now, in beautiful Kanata, Ontario, just outside the nation’s capital. Make plans now to join us at the Comfort Inn Ottawa West the afternoon of Tuesday, September 18, 2018 for a series of talks focused on the concerns of Canadian mefloquine veterans and Canadian healthcare providers caring for these veterans. Topics will include an update on the status of current VAC claims related to mefloquine poisoning and factors to consider when filing a VAC claim, including those conditions that can be linked to mefloquine exposure. The meeting will also discuss use of the foundation’s WRMI-2 screening instrument for symptomatic mefloquine exposure, and how clinicians and veterans can improve documentation supporting their claim.
 The meeting is scheduled to coincide with the annual Canadian mefloquine awareness event on the front steps of Parliament Hill in Ottawa, Ontario, Wednesday, September 19, 2018 at 1 p.m., hosted by advisory committee member Marj Matchee. Echoing calls from Canadian veterans from last’s year’s event, the foundation has called upon the Canadian government to reopen the Somalia Commission of Inquiry to investigate the role of mefloquine in the events of that era, as described more fully in this press release. Canadian veterans will also be calling on the Canadian government to acknowledge chronic disability from mefloquine poisoning, and to support outreach and research. The Quinism Foundation thanks the Royal Canadian Legion for their continued support of calls for further research into mefloquine poisoning.
The meeting is scheduled to coincide with the annual Canadian mefloquine awareness event on the front steps of Parliament Hill in Ottawa, Ontario, Wednesday, September 19, 2018 at 1 p.m., hosted by advisory committee member Marj Matchee. Echoing calls from Canadian veterans from last’s year’s event, the foundation has called upon the Canadian government to reopen the Somalia Commission of Inquiry to investigate the role of mefloquine in the events of that era, as described more fully in this press release. Canadian veterans will also be calling on the Canadian government to acknowledge chronic disability from mefloquine poisoning, and to support outreach and research. The Quinism Foundation thanks the Royal Canadian Legion for their continued support of calls for further research into mefloquine poisoning.
Discounted non-smoking hotel rooms at the Comfort Inn Ottawa West are available for up to three nights September 17 through September 20, 2018, featuring 2 double beds or 1 queen bed, for just $139.99 a night plus applicable taxes, by calling the hotel group sales office at 613-592-2200 extension 7361 and quoting the booking code QUINISM FOUNDATION. This special low rate expires in one week, so book now! If you would like more information about the rally, please contact Marj Matchee directly. To secure attendance at the educational meeting itself, please be sure to reserve your space by confirming your attendance with The Quinism Foundation. Remember, space is extremely limited, so please make your plans now!
Charitable Donations
The Quinism Foundation relies entirely on private donations for its operations. If you have not yet made a charitable donation, please consider making one today to support the charity of your choice. The foundation is proud to be listed as a registered charity in the PayPal Giving Fund. You can also create a Facebook fundraiser to encourage your friends to support your selected charity. 100% of donations made to these funds support the charity of your choice. As well, when you shop Amazon Prime using this link Amazon will donate 0.5% the price of eligible AmazonSmile purchases to The Quinism Foundation. The foundation is a registered 501(c)(3) non-profit charity. You can read more about the foundation’s non-profit status by reviewing our listing on Guidestar.
Tafenoquine Approved for Prevention — With Significant Warnings
Following the foundation’s appearance at an FDA advisory committee meeting at which we warned of the drug’s risks, “the next mefloquine”, a neurotoxic quinoline drug called tafenoquine (to be marketed as Arakoda™) has been approved by the FDA with significant warnings, following a highly divided 9-4 vote on its safety. As with mefloquine before it, FDA is now warning that the most common symptoms with use of tafenoquine include “insomnia, depression, abnormal dreams and anxiety”, and is requiring that patients prescribed tafenoquine for prevention of malaria be warned that nightmares, insomnia, anxiety, or changes in mood lasting 3 days or longer require the patient to contact their healthcare provider.
The foundation believes these warnings will preclude the safe use of tafenoquine for many travelers, including military personnel (among whom these symptoms are highly prevalent). The foundation also believes that these warnings are a tacit acknowledgment by FDA of the foundation’s concerns, articulated at the FDA advisory committee meeting and in regulatory submissions and public correspondence, that tafenoquine shares the same neurotoxic liability as mefloquine, and that, as with mefloquine, these symptoms must be considered prodromal to more serious neurotoxic effects with continued use of the drug.
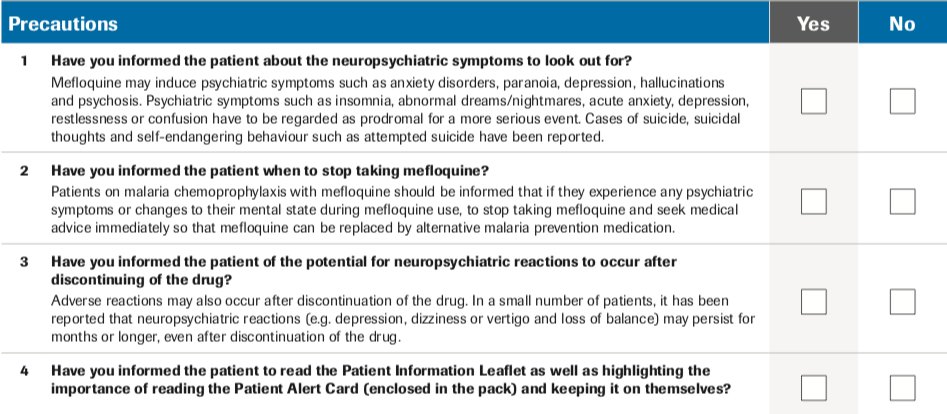
Tellingly, FDA’s approval of tafenoquine comes only weeks after drug regulators in Europe finally required, more than 30 years following the initial approval of mefloquine, that prescribers complete a check-list confirming they have warned patients of the need to discontinue the drug at the onset of similar symptoms.
In the coming months, the foundation will be corresponding with the FDA, the Centers for Disease Control and Prevention (CDC), the Department of Defense (DoD), and various travel medicine organizations and clinics to ensure that warnings related to tafenoquine and mefloquine are properly communicated. The foundation also encourages consumers prescribed tafenoquine and mefloquine who experience any adverse effects, including abnormal dreams or nightmares and insomnia, to report these to the FDA Medwatch program. The foundation also reminds consumers who continue to experience adverse effects that continue over six months since their last report, to file updated FDA Medwatch reports describing the continued chronic nature of their symptoms.
Help Us Grow our Email and Social Media Presence
Please help the foundation grow its email and social media presence by encouraging your followers to sign-up for our email list, and to follow the foundation on Twitter and Facebook.